In addition it allows the determination of FLUAV subtype H1 H3 and H1pdm09. In anticipation of similar challenges for all respiratory virus reagents we performed a diagnostic comparison study of the Hologic Panther Fusion RT-PCR Respiratory assays Fusion RA with the GenMark ePlex Respiratory Pathogen Panel ePlex RPP.
 German Lab Demonstrates Feasibility To Detect Sars Coronavirus 2 Sars Cov 2 Using Hologic S Panther Fusion System Business Wire
German Lab Demonstrates Feasibility To Detect Sars Coronavirus 2 Sars Cov 2 Using Hologic S Panther Fusion System Business Wire
Video introducing the Panther Fusion system evolution of the Panther system and system featuresbenefits.

Hologic respiratory panel. Molecular tests are the best detection option due to their high sensitivity and specificity. International Modern Slavery Statement. The foundation for assay consolidation future scalability and growth.
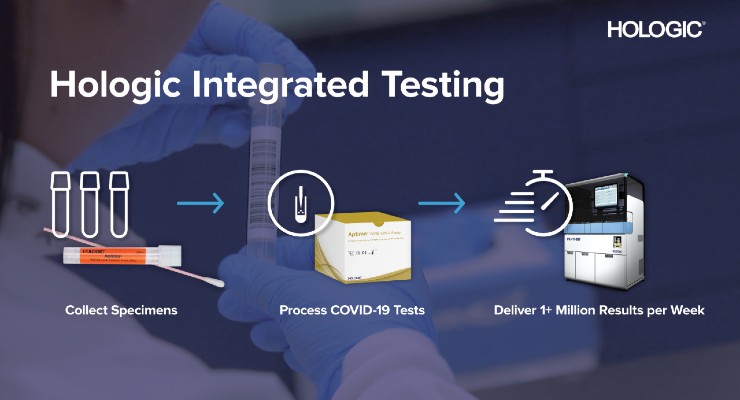
AdvaMed Code of Ethics. 4 Additionally the Hologic SARS-CoV-2 assays were the first molecular diagnostic tests specifically authorized for use in both symptomatic and asymptomatic people. Add the Panther system to your lab and the capability to consolidate menu on a fully automated system load samples in any order at any time eliminate batch constraints and decrease turnaround time.
Combined SARS-CoV-2 nucleic acid amplification testing and respiratory virus panel RT-PCR on the Hologic Panther Fusion system. San diego CA 92121. Unsere 3 Kerngeschäftsbereiche konzentrieren sich auf die Diagnostik das Imaging und die gynäkologische Chirurgie.
The Panther Fusion system is able to process up to 335 respiratory tests in 8 hours. Hologic Panther Fusion PCR. Online ahead of print.
To evaluate the performance of the Hologic Panther Fusion Respiratory Assays RA compared to the GenMark ePlex Respiratory Pathogen Panel RPP and to assess the ability of the Panther Fusion to perform parallel testing of SARS-CoV-2 and other respiratory. Order Online iStore Contact our Compliance Hotline. The Panther Fusion system has competitive throughput utilizing less space than many competitor platforms.
Seegenes Allplex Respiratory Panel 1 Allplex RP1 is a real-time one-step RT-PCR assay for the simultaneous detection of FLUAV FLUBV HRSV-A and HRSV-B. 10210 GENETIC CENTER DRIVE. Run independently or as part of a panel on the Panther Fusion system with true patient-specific results.
Respiratory Infectious Diseases Hologic. The Panther Fusion Respiratory menu available in the US allows for syndromic respiratory testing for the most common respiratory viruses including flu A and B RSV AdV hMPV RV and parainfluenza 1-4. The Panther Fusion Respiratory menu leverages a legacy of expertise in respiratory viral molecular diagnostics from Hologic that began over a decade ago with the Prodesse Plus Series of respiratory assays.
Three respiratory assays comprise the IVD respiratory testing menu on the fully automated Panther Fusion system. HOLOGIC ist ein führendes Unternehmen im Bereich der Entwicklung Herstellung und dem Vertrieb von hochwertigen diagnostischen Produkten medizinischen Bildgebungssystemen und chirurgischen Artikeln mit Schwerpunkt in der Frauenheilkunde. This article was originally published here.
This is an interesting contrast to the larger respiratory panels of BioFire and Luminex each of which detects 20 targets or Nanosphere s test which can detect 16 targets as either a full multiplex panel or in user-defined subsets. Third Respiratory Assay Now FDA Cleared on Hologics New Panther Fusion System -- New Assay Detects Adenovirus Human Metapneumovirus and Rhinovirus ---- Modular Panels Including Influenza RSV. In addition we evaluated and optimized combined testing of all three Fusion Respiratory assays with SARS-CoV-2 nucleic acid.
In this study performance characteristics of two commercial methods the Panther Fusion assay Hologic Inc San Diego CA USA were compared to Allplex respiratory panels Seegene Seoul South Korea for the detection of influenza A Flu A influenza B Flu B respiratory syncytial virus RSV parainfluenza virus PIV human metapneumovirus hMPV rhinovirus RV and adenovirus. A recent report published by the FDA compared more than 50 COVID-19 molecular tests and demonstrated that Hologics assays are the most analytically sensitive fully automated high-throughput molecular tests on the market. Hologics flu and respiratory assays enable customers to run only the targets requested for a patient allowing for personalized testing and helping them control costs within their laboratories.
510 k Number. Breakthrough Diagnostic Medical Imaging Solutions. Combine Panther Fusion and Aptima tests and process up to 500 tests in 8 hours.
Each is a multiplex real-time PCR in vitro diagnostic test. For the first assays Hologic has chosen to develop small multiplex respiratory viral panels.
![]() Syndromic Respiratory Testing Hologic Inc Panther Fusion Respiratory Assays Us
Syndromic Respiratory Testing Hologic Inc Panther Fusion Respiratory Assays Us
 Flu And Respiratory Assays Ce Marked In Europe Clinical Laboratory Int
Flu And Respiratory Assays Ce Marked In Europe Clinical Laboratory Int
 Respiratory Assays Clinical Lab Products
Respiratory Assays Clinical Lab Products
 Hologic Launches Second High Throughput Molecular Assay For Novel Coronavirus Covid 19 Labmedica Com
Hologic Launches Second High Throughput Molecular Assay For Novel Coronavirus Covid 19 Labmedica Com
 Sars Cov 2 Assays Aptima Panther Fusion Hologic
Sars Cov 2 Assays Aptima Panther Fusion Hologic
 Hologic S Panther Fusion System Shows Feasibility For Detection Of Sars Coronavirus 2 Sars Cov 2 Covid 19 Labmedica Com
Hologic S Panther Fusion System Shows Feasibility For Detection Of Sars Coronavirus 2 Sars Cov 2 Covid 19 Labmedica Com
 Sars Cov 2 Assays Aptima Panther Fusion Hologic
Sars Cov 2 Assays Aptima Panther Fusion Hologic
 Panther Fusion Assays Hologic Inc
Panther Fusion Assays Hologic Inc
 Hologic To Release Second High Throughput Sars Cov 2 Molecular Assay
Hologic To Release Second High Throughput Sars Cov 2 Molecular Assay
Panther Fusion System Hologic Inc Us Molecular Testing Instrumentation
 Sars Cov 2 Assays Aptima Panther Fusion Hologic
Sars Cov 2 Assays Aptima Panther Fusion Hologic
 Hologic S New Panther Fusion System Flu And Respiratory Assays Now Ce Marked In Europe Healthmanagement Org
Hologic S New Panther Fusion System Flu And Respiratory Assays Now Ce Marked In Europe Healthmanagement Org
 Sars Cov 2 Assays Aptima Panther Fusion Hologic
Sars Cov 2 Assays Aptima Panther Fusion Hologic
 Hologic Inc Third Respiratory Assay Now Fda Cleared On Hologic S New Panther Fusion System
Hologic Inc Third Respiratory Assay Now Fda Cleared On Hologic S New Panther Fusion System

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.